Abstract
BACKGROUND: High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) is now the standard of care for newly diagnosed MM and refractory DLBCL patients. However, relapse remains the primary cause of morbidity and mortality, and thus, new therapeutic strategies are required to improve overall survival. In recent years, treatment with immune checkpoint inhibitors Nivolumab (Nivo), an anti-PD1 mAb, or Ipilimumab (Ipi), an anti-CTLA4 mAb, as single agents have achieved durable responses in patients with advanced solid tumors, and may prove to be useful for the treatment of hematological malignancies.
RATIONALE: In patients with advanced cancers including melanoma and Hodgkin's lymphoma, combined Nivo/Ipi therapy resulted in superior clinical efficacy compared to monotherapy. The immediate post-ASCT period provides an ideal window to introduce combined therapy because disease burden is at a minimum and the conditioning-induced inflammatory environment may enhance checkpoint inhibition effects on anti-tumor immune responses.
OBJECTIVE: To study the Treg and memory T cell compartments in the peripheral blood (PB) of high-risk MM and DLBCL patients, within the first 12 wks post-ASCT, during a Nivo/Ipi treatment regimen.
STUDY DESIGN: The interim longitudinal analyses of early Treg and memory T cell profiles was performed on PB sampled from patients up to 12 wks post-ASCT and treated with Nivo + Ipi, with a focus on four patient disease groups: A=de novo DLBCL; B=recurrent high-risk DLBCL; E=de novo high-risk MM; and F=recurrent MM. PB samples obtained from patients at initial screening (PB0) served as a baseline. The MM patients underwent conditioning with CDE or melphalan 200 mg/m2 followed by mobilization with filgrastim, apheresis, and hematopoietic progenitor cell reinfusion. Lymphoma patients were conditioned with BEAM. Starting 2-4 wks later, they were administered double therapy (Ipi 1mg/kg; 6 doses at wks 1, 4, 7, 10, 16, 22 and Nivo 3 mg/kg; 12 doses at wks 1, 4, 7, 10, 12, 14, 16, 18, 20, 22, 24, 26). PB was collected just prior to any treatment at various time points.
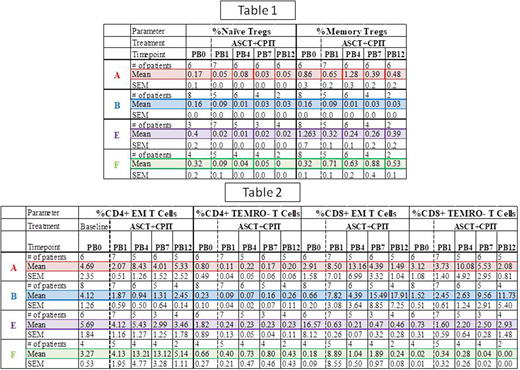
RESULTS:Table 1 summarizes the mean ± sem % of total lymphocytes for the Treg compartment at all time points. The % of naïve (RO-) Tregs was decreased up to wk 12 in all the groups (A; PB0 0.17; PB12 0.05), (B; PB0 0.16; PB12 0.03), (E; PB0 0.40; PB12 0.02), (F; PB0 0.32; PB12 0.00); and in memory (RO+) Tregs, except for F, (A; PB0 0.86; PB12 0.48), (B; PB0 0.16; PB12 0.03), (E; PB0 1.26; PB12 0.39), (F; PB0 0.32; PB12 0.53). Similar results were observed in the absolute numbers of Tregs for all groups.
In the memory T cell compartment, summarized in Table 2, by wk 12, the % effector memory (EM) CD4+ T cells increased in the A and F groups, but were decreased in B and E (A; PB0 4.69; PB12 5.33), (B; PB0 4.12; PB12 2.45), (E; PB0 5.69; PB12 2.99), (F; PB0 3.27; PB12 5.14). In contrast, EM CD8+ T cells in the A and E groups decreased in this compartment (A; PB0 2.91; PB12 1.49) (E; PB0 16.57; PB12 0.46), while the B group markedly increased by wk 12 (B; PB0 0.65; PB12 17.91), and the F group remained equivalent (F; PB0 0.18; PB12 0.24). Moreover, the centralmemory (CM) CD4+ T cell compartment was moderately decreased in all groups, except the F group (A; PB0 2.59; PB12 1.04), (B; PB0 4.12; PB12 2.5), (E; PB0 5.6; PB12 0.82), (F; PB0 1.62; PB12 1.5). Finally, the % of CD4+CCR7+RO-terminally differentiated (TEMRO-) cells was decreased in A, E and F, but not in B, groups (A; PB0 0.80; PB12 0.20), (B; PB0 0.23; PB12 0.26), (E; PB0 1.82; PB12 0.23), (F; PB0 0.66; PB12 0.43). In contrast, the % of CD8+CCR7+RO- TEMRO- cells was increased in B (PB0 1.52; PB12 11.73) and E (PB0 0.76; PB12 2.93), but not in A (PB0 3.12; PB12 2.08) and F (PB0 0.02; PB12 0.0.00) groups. Similar trends were observed with the absolute numbers of subsets.
CONCLUSIONS: Combined Nivo + Ipi therapy administered early post-ASCT exhibited a trend of decreased presence of naïve Tregs, in all groups, and memory Tregs in all groups but F by wk 12. In addition, there was an increased trend in the presence of CD8+ EM T cells in group B, and of the CD4+ EM T cells in groups A and F. Increased EM activity could potentially enable the immune system to continue to target tumor cells while in therapy. Finally, there was an increased trend in both CD4+ and CD8+ TEMRO- T cells in group B, which were consistent with the notion of exhaustion of longer surviving EM T cells.
Munshi:Kite: Speakers Bureau. Atkins:Merck: Consultancy; BMS: Consultancy. Biran:Takeda: Consultancy, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Merck: Research Funding; BMS: Research Funding; Amgen: Consultancy, Speakers Bureau. Siegel:Takeda: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Merck: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria; Novartis: Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau. Feldman:Portola: Research Funding; Seattle Genetics: Research Funding, Speakers Bureau; Johnson and Johnson: Speakers Bureau; KITE: Speakers Bureau; Pharmacyclics: Speakers Bureau; Janssen: Speakers Bureau; Celgene: Speakers Bureau. Skarbnik:Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Gilead Sciences: Honoraria, Speakers Bureau; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Honoraria, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.